
#Gamp 4 and gamp 5 guidelines. software
They have extensive experience operating in the life sciences sector validating software systems and automation to FDA Standards – you can be assured that your software and equipment will be validated to the highest industry standards by our skilled engineers. The Dataworks team of validation engineer’s work with GAMP 5 guidelines.

This is where the experience and reputation of your validation engineers count. An engineer who takes a measured approach is also required when undertaking the risk assessment to avoid completing too much or too little validation on each project.

Why your Validation Engineers should work within GAMP GuidelinesĪs GAMP is a guideline and not an industry regulation it is important to ensure that your validation engineers are up to date with the latest revisions and working to the highest industry standards available. It is then used to determine if validation is required and how much validation is required based on the the level of risk and system impact. Risk Assessment is used to identify, evaluate, control and review the risks.
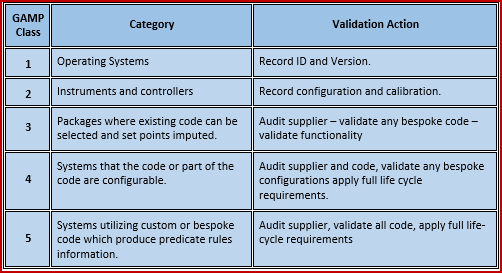
For this reason the validation effort should be based on the risk impact/assessment as well as taking into account the above guidelines. As a result of this too much or too little validation effort may be executed. Oftentimes there can be some ambiguity as to which category a software application falls under. The GAMP Software categories can be open to interpretation. This software could be written in-house and is possibly the highest risk of the software categories as it is customised and there is a higher level risk of errors within the application code. Is software that is generally written from scratch to fulfil the business need. GAMP Software Category 5 – Bespoke software.As a result of this GAMP Category 4 software can require a much higher level of validation than GAMP Category 3. The functionality of the software can be configured to return different outputs depending on the configuration. This is possibly the biggest and most complex category. – GAMP Category 4 software is software applications that are configured to meet user specific business needs. GAMP Software Category 4 – Configurable Software.This category also covers cases where a configurable software product is being used but only with the default configuration. An example of a GAMP category 3 system that is provided with computerised controllers, would be Programmable Logic Controllers (PLC’s) where the application cannot be changed although it may be parameterised to meet the business need. The software is capable of operating and automating the business process without any modification. Non Configurable Software is sometimes called COTS (Commercial-Off-The-Shelf-Software) or just OTS (Off-The-Shelf-Software). – There is no fixed rule as to the validation approach for GAMP Category 3 systems. GAMP Software Category 3 – Non Configurable Software Configuration.The validation is performed on the hosted application not on the infrastructure. Infrastructure is qualified but not validated. Infrastructure software in its most simple form is the operating system on which the application software resides. – unless a very simple control system (PLC and HMI) there is likely to be some elements of infrastucture software. GAMP Software Category 1 – Infrastructure Software.Note: In GAMP 4 there were five software categories these were revised in GAMP 5 to four categories. The following are the Process Control Systems GAMP 5 Software Categories: Categorising software is used to support the approach to validation based on the difficulty and individuality of the computerised system.

These documents then form the basis for the traceability matrix and for the formal testing of Internal Acceptance, Factory Acceptance, and Site Acceptance. The process begins with a User Requirements Specification for the machine, from which a Functional Requirement and a Design Specification are created. It is a formal process of thorough documentation, testing, and logical process steps that validate clients’ required specifications. When GAMP is adhered to, clients in the medical device, pharmaceutical and life sciences industries are assured that their machines are designed and built under a Total Quality Management System. All aspects of production from the raw materials, facility and equipment to the training and hygiene of staff are covered by GAMP recommendations. ‘Quality by design’ is a central principle of GAMP and advocates that quality is built into each stage of the manufacturing process.


 0 kommentar(er)
0 kommentar(er)
